Researchers from South Korea have created a novel technique for generating hydrogen from sugarcane, a fuel considered the future of energy.
This innovative technology utilizes the energy from the sun along with an unexpected resource: residue from sugarcane.
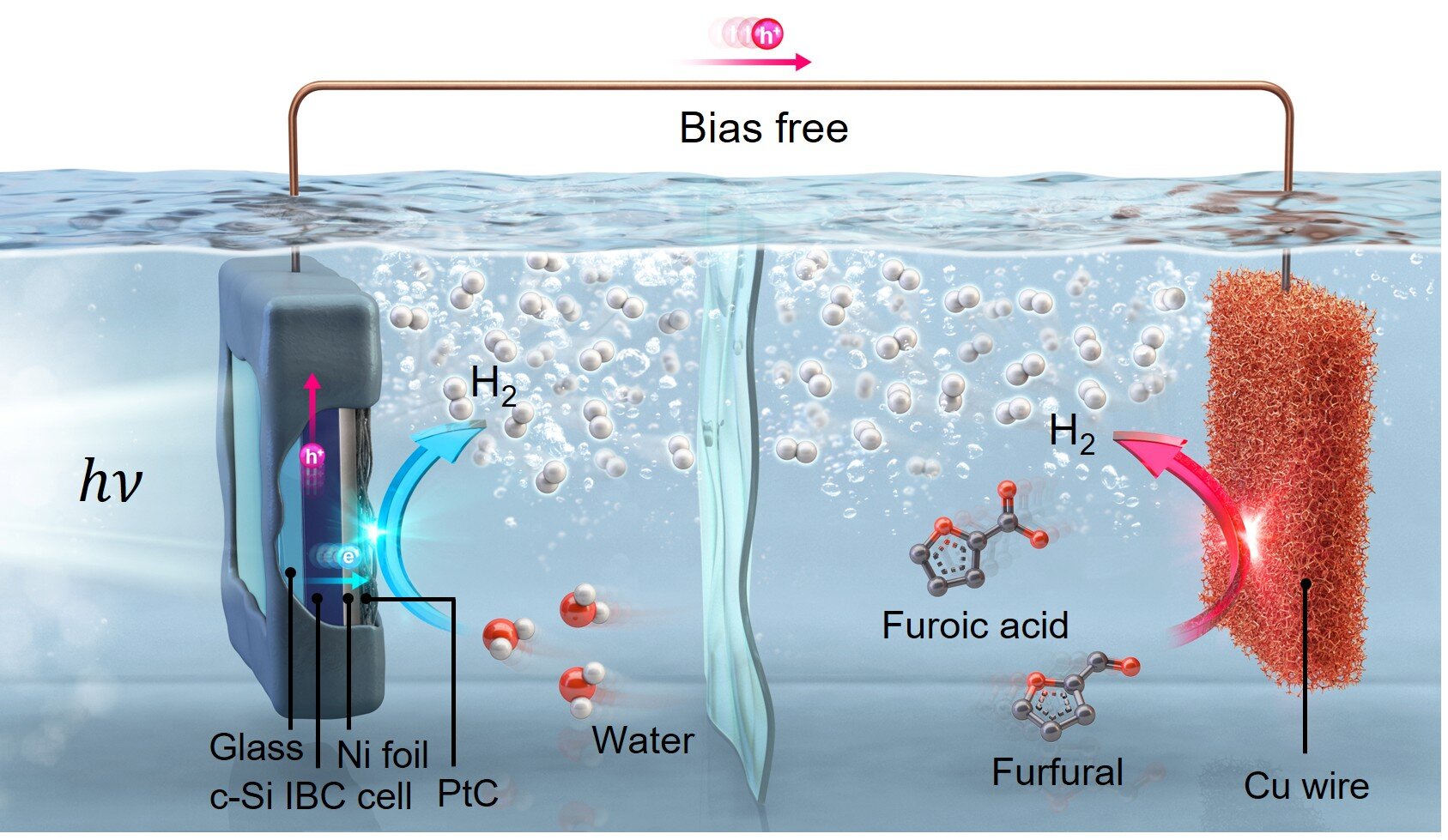
The study presents a novel photoelectrochemical system utilizing furfural, an important chemical derived from sugarcane residues.
“This groundbreaking technique employs biomass sourced from sugarcane residues along with silicon photoelectrodes to produce H2 solely through sunlight exposure, reaching a yield rate quadruple the standard established for commercial application by the U.S. Department of Energy (DOE),” according to a press release from the Ulsan National Institute of Science & Technology.
Sugarcane production method
Hydrogen
(H2) is regarded as a next-generation fuel due to its clean combustion, which produces zero greenhouse gases. Additionally, it boasts a high energy storage density, approximately 2.7 times greater than that of gasoline.
Nevertheless, much of the hydrogen (H2) generated originates from natural gas, leading to significant emissions of heat-trapping carbon dioxide.
To address these problems, the group developed an environmentally friendly photoelectrochemical (PEC) hydrogen production system.
This cutting-edge system utilizes sunlight to drive a dual-stage process.
At one copper electrode, furfural undergoes oxidation to generate hydrogen. Notably, this process concurrently yields furoic acid, which is a valuable secondary product.
At the same time, water is broken down at the second silicon photoelectrode, producing hydrogen. This two-pronged generation process is what makes them exceptionally efficient.
The system generates a hydrogen output of 1.4 millimoles per square centimeter per hour.
“This two-part production process theoretically increases the output rate twice as much as traditional PEC systems, achieving an efficiency of 1.4 mmol/cm²·h, which is almost quadruple the U.S. Department of Energy’s benchmark of 0.36 mmol/cm²·h,” according to the research team.
explained
.
Submerged design
The secret to this efficiency stems from the way the system handles energy management.
Upon striking the silicon photoelectrode, sunlight produces electrons.
The process of generating hydrogen (H2) starts when the photoelectrode captures sunlight and releases electrons. According to the team, crystalline silicon is optimal for yielding numerous electrons.
Nevertheless, there is one hurdle. The voltage produced is usually quite low, making it challenging to initiate hydrogen generation without an additional power supply.
The team addressed this issue by implementing a furfural oxidation reaction at the opposite electrode. This process helps balance the system’s voltage.
They additionally shielded the electrode with protective layers of nickel foil and glass to ensure the system remains stable over time within the electrolyte.
Notably, the UNIST team’s silicon photoelectrode features a “submerged design” which inherently provides cooling, thereby enhancing the overall system’s efficiency.
This new development marks a major step towards a
sustainable hydrogen economy
By utilizing copious amounts of organic matter and solar power, this approach has the potential to help nations generate more affordable and environmentally friendly hydrogen fuel.
Professor Ji-Wook Jang indicated that this technology has the potential to boost “the economic feasibility of solar hydrogen production” and secure competitiveness with respect to fossil-fuel derived hydrogen in terms of cost.
The research was published in the journal
Nature Communications.
